How Bacteria Learned to Make Human Insulin - and Opened the Door to Modern Biotech
The Insulin Problem
Back in the 1970s, people with type 1 diabetes, whose pancreas produces little to no insulin, had pretty limited choices. Most of the time, they had to rely on insulin that was extracted from the pancreases of pigs and cows.
For some, this worked well enough, but for others, it was a constant source of worry. The animal insulin wasn’t exactly the same as human insulin. Even slight differences in amino acid sequences could cause some patients to have immune reactions, such as itching, swelling, rashes, and in more serious cases, anaphylaxis; a severe and potentially life-threatening allergic reaction.
To make matters worse, over time, the body could start producing antibodies against the foreign insulin, making it less effective. For those patients, every shot was a bit of a gamble of keeping them alive, or making things worse.1
This essay is based largely on the book Genentech: The Beginnings of Biotech by Sally Smith Hughes, which tells a story of how Genentech(pronounced JEN-en-tek) produced human insulin at scale to treat people with type 1 diabetes.
But this isn’t just about insulin. It’s a story of how biology became something we could engineer.
Recombinant DNA: A Scientific Breakthrough
To solve the insulin problem, there had to be a scientific breakthrough. That path opened up in the early 1970s when two scientists, Herbert Boyer and Stanley Cohen, pioneered a method for combining DNA from different organisms to create new genetic sequences.
Although they were both exploring how DNA could be exchanged and recombined between different organisms, their focus areas were different, and it's fascinating how their work complemented each other to invent what's known as recombinant DNA today.
Herbert Boyer and Restriction Enzymes
Herbert Boyer’s journey into scientific discovery started off in an unexpected way. After getting turned down by medical school, he decided to dive into graduate studies in bacterial genetics, and that detour ended up being important.
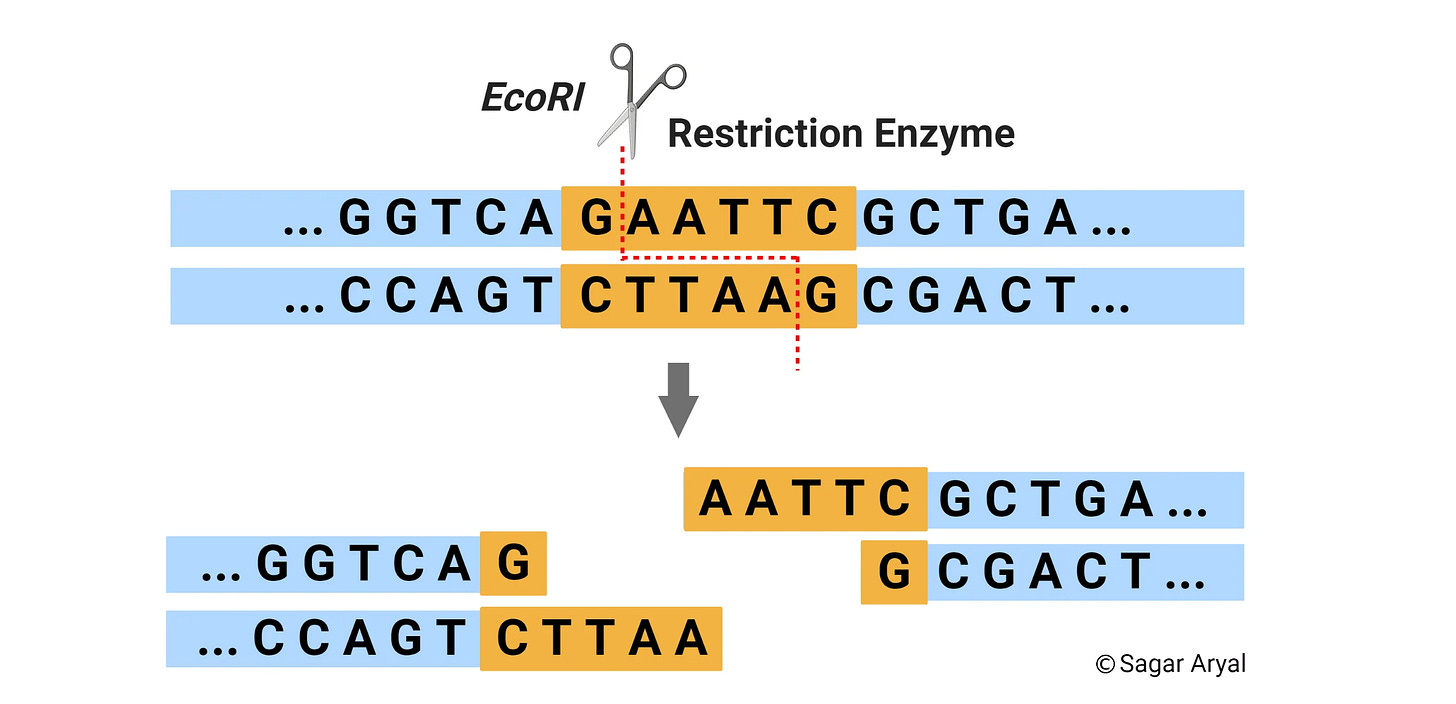
While doing his postdoc at Yale, Boyer really got into studying genetic exchange in bacteria. He became particularly intrigued by restriction enzymes, which cut DNA at specific spots.
These restriction enzymes are naturally found in bacterial cells. Bacteria use them to chop up foreign virus DNA, which helps stop the virus from multiplying inside the bacteria.
Back in the 1960s, scientists were just starting to realize that different restriction enzymes cut DNA at unique and predictable sites. Boyer was fascinated by how precise and powerful these enzymes could be.
Eventually, his lab managed to isolate a specific restriction enzyme called EcoRI, which would go on to become widely used. This enzyme cuts DNA consistently at a certain location, as shown below.2
Boyer’s work on restriction enzymes was great for cutting DNA accurately, but it needed something else to achieve genetic exchange and recombination: a way to carry and replicate foreign DNA inside cells. That’s where Stanley Cohen stepped in.
Stanley Cohen and Plasmids
Stanley Cohen took a more traditional path into the scientific field. After getting his medical degree, he jumped into molecular biology during his postdoctoral work and eventually focused on plasmids.3
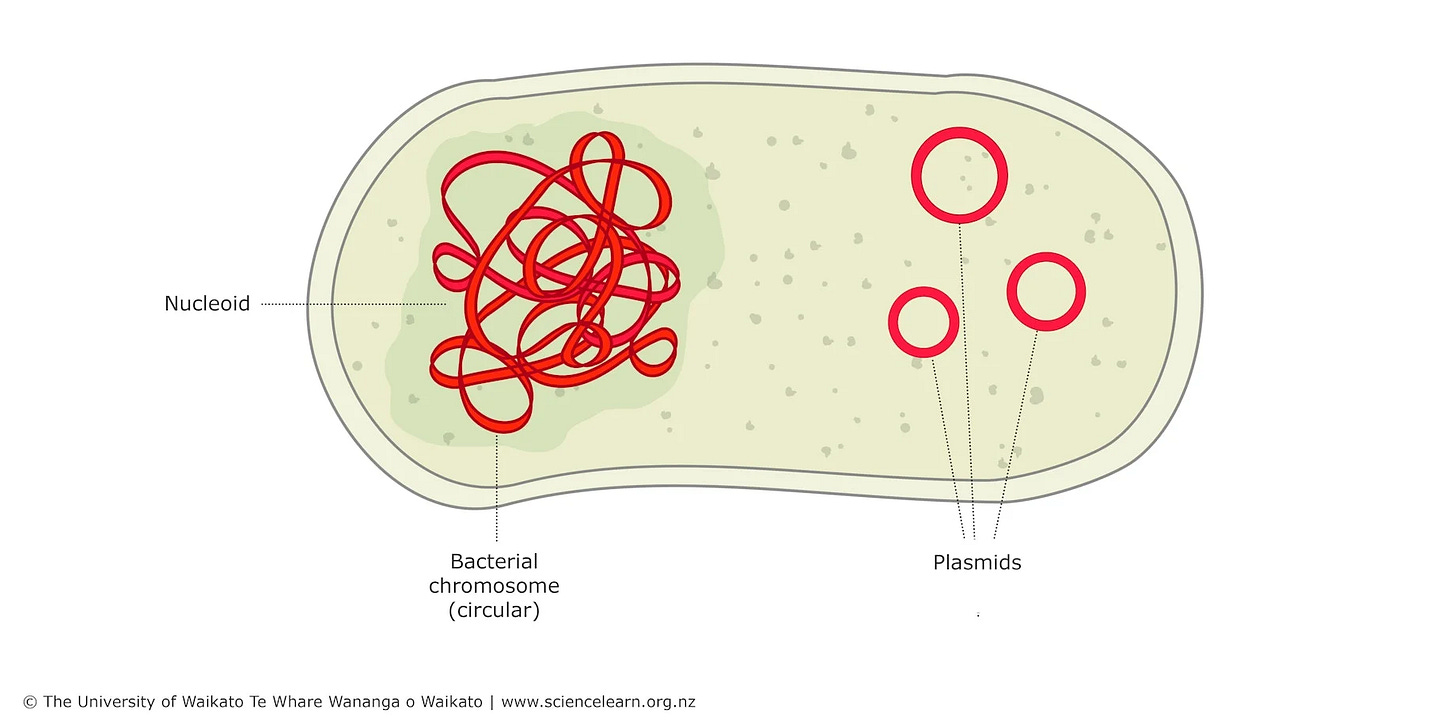
Plasmids are these little loops of DNA found in bacteria that can give them some pretty cool abilities, like resisting certain antibiotics or using unique food sources.
You can think of plasmids as tiny bonus instruction booklets inside bacteria. They’re separate from the main instructions, which are in the chromosomal DNA. While chromosomal DNA can have millions or even billions of bases, plasmids usually only have a few thousand at most.
Thanks to their small size, circular shape, and ability to replicate independently of the bacterial chromosome, plasmids are stable and easy to manipulate.
By the early 1970s, Cohen figured out a way to isolate plasmid DNA from bacterial cells. He would break it into pieces and then put those pieces back into the bacteria to test if it could be used for genetic recombination.
But his method was unfortunately pretty slow and inefficient; the DNA ended up getting fragmented randomly, and it was tough for the bacteria to take it up.
Still, he published numerous papers on plasmids and was recognized as a leader in the field. In 1972, Cohen began organizing a conference on plasmid research, eager to explore better methods for manipulating plasmid DNA.
Their Collaboration and the Birth of Recombinant DNA
It was at the plasmid conference in Honolulu where Cohen and Boyer first met. Cohen had heard about Boyer's unpublished research on EcoRI and invited him to speak at the event.
As Boyer explained how EcoRI could cut DNA in a precise and predictable way, Cohen immediately recognized how this could change his work with plasmids. If they could use EcoRI to make clean cuts in a plasmid like molecular scissors and then insert foreign DNA, they could create a hybrid plasmid that could be introduced into bacteria. This would make DNA recombination much more reliable and efficient.
The idea was exciting. Cohen approached Boyer with a proposal for collaboration. They quickly figured out their roles: Cohen's lab would focus on isolating and transferring plasmids, while Boyer’s team would handle the enzymatic side of things.
By March of the following year, they were celebrating their achievements. They had successfully created hybrid plasmids; DNA fragments from different sources had been joined together. They also realized that they had not only combined DNA from different sources but had also successfully made exact copies of the recombinant DNA inside bacterial cells.
But scientific discovery alone doesn’t change the world. For that, someone had to turn this potential into a product.
Genesis of Genentech
Robert Arthur Swanson, a Kleiner Perkins venture capitalist, first heard of recombinant DNA in one of the meetings with their portfolio company. Most people in the meeting didn't understand it, but he, as a chemistry major in college, said, "It is revolutionary; it will change the world; it’s the most important thing I have ever heard."
Swanson saw how this scientific breakthrough could be used to create practical products, particularly medicines, and he believed the timing was right to commercialize it.
In 1976, after leaving Kleiner Perkins, Swanson reached out to Herbert Boyer to explore the idea of forming a company around it. Although Boyer understood the practical applications of their work, he wasn't inclined to start a business. Swanson, however, was persistent. He arranged a meeting with Boyer to discuss how recombinant DNA could be used commercially.
What was meant to be a ten-minute meeting turned into a three-hour conversation and at least as many beers. They found themselves compatible, bonded by enthusiasm for the technology and a shared down-to-earth style.
That long conversation shifted something; what was once academic curiosity now had a commercial trajectory. Together, they would end up forming Genentech, the first company dedicated to using recombinant DNA to develop medicines.
The true power of recombinant DNA lies not only in recombining pieces of DNA and cloning them in a microorganism like bacteria. The power lies in the fact that the microorganism can express the DNA of another being as a functional product, such as a protein, from which you can produce medicines.
But turning recombinant DNA from an idea into a medicine wouldn’t be easy. Genentech had no lab of its own. They just had ambition back then: to prove that engineered microbes could reliably produce a functional product.
Swanson and Boyer agreed to choose human insulin as the ultimate target because it was medically important and scientifically feasible. The sequence of amino acids making up the human insulin protein was already known. On top of that, it was a relatively small protein, only fifty-one amino acids long, which made it easier to synthesize.
That said, even insulin was a complex molecule for a first attempt. To make sure the approach would work, they needed to start even smaller.
Making Somatostatin
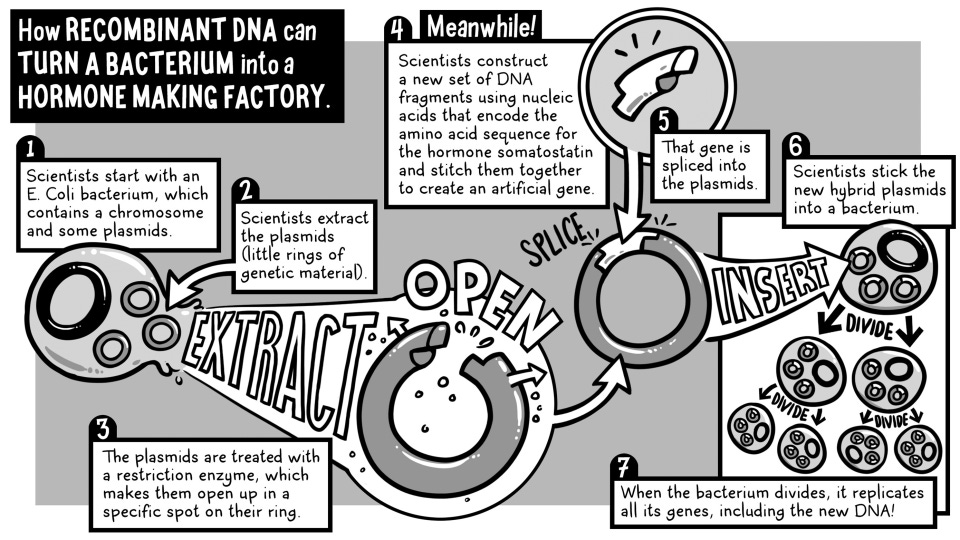
So the Genentech team decided to start with somatostatin, a small hormone made of just 14 amino acids.
Here was the plan: they isolated plasmids from E. coli bacteria and cut a specific spot of the plasmids with a restriction enzyme. Meanwhile, they chemically synthesized the DNA sequence that carries the instructions for making somatostatin. Once DNA synthesis was complete, the synthesized gene was spliced into the plasmids. Then they inserted the new recombinant plasmids into a bacterium.
The above image is a more detailed explanation of the plan from a Genentech's blog post.
Once the recombinant plasmid was introduced into a bacterial cell, the idea was that the bacteria would read the new gene and start producing somatostatin. If that worked, it would prove that even a tiny, human-designed gene could be expressed by bacteria to make a real, functional protein.
At this point, it was still unclear if bacteria could read the genes of higher organisms(somatostatin is a human peptide hormone) and express them as proteins. They were about to find out.
Boyer called scientists at City of Hope, including Keiichi Itakura and Arthur Riggs, and they agreed to build the gene for somatostatin from scratch by stitching together short pieces of synthetic DNA. DNA synthesis might sound simple (just string A, T, C, and G together, right?), but in reality, it’s surprisingly hard, especially back then. It has to be done precisely; errors are common, and it is very sensitive. It was slow, frustrating work.
Things picked up when Roberto Crea, a postdoc from Italy, joined and brought some serious DNA chemistry skills. He used a fancy new machine, a high-performance liquid chromatograph, to clean up the gene fragments, making them much more accurate. The team then pieced the fragments together into a full gene and inserted it into a bacterial plasmid.
At first, nothing happened. The test came back negative; there was no somatostatin at all. The team figured the bacteria were producing it, but their own enzymes were destroying it right away. So, they came up with a smart solution: they attached the somatostatin gene to a piece of a bigger bacterial protein, hoping it would offer some protection. Once it was made, they could chemically separate the hormone. And guess what? That trick actually worked.
In August 1977, they reran the experiment, and this time the results were crystal clear: the bacteria had made somatostatin! It was the first time anyone had used a synthetic gene to get bacteria to produce a real mammalian protein. That moment proved the whole idea could work, and it gave them the green light to tackle insulin next.
From Somatostatin to Insulin
Genentech's initial win with somatostatin showed that bacteria could be engineered to produce human proteins, but insulin was a different game. The insulin molecule was more complex, and they needed to make it in large quantities to help millions of people. The whole process had to be robust, reliable, and safe; there was no room for mistakes when lives were at stake.
So, it took another five years of hard work. In 1982, six years after Boyer and Swanson first connected, the FDA gave the green light to Humulin. This was the first-ever recombinant DNA drug; human insulin made by genetically modified bacteria. It was safe, pure, and for the first time, it was just like the insulin our bodies naturally produce.
For people with type 1 diabetes, this was about more than just convenience. It was about freedom from unpredictability, immune reactions, and the constant fear that each injection might cause more harm than good. With recombinant insulin, treatment became safer, more consistent, and truly human. No longer was every dose a gamble. It became a therapy they could rely on.
That breakthrough was only the beginning. Today, recombinant DNA is the backbone of countless therapies, from insulin and growth hormone to cancer-fighting monoclonal antibodies. The same tools Genentech helped pioneer are now used far beyond medicine. In food production, for example, scientists engineer microbes to produce everything from dairy proteins to meat alternatives. The core idea remains the same: design a gene with instructions, insert it into a host organism, and let the cell’s machinery make the target product.
Another downside, obviously, is the exploitation of other animals. Eli Lilly, a manufacturer of insulin back then, needed 56 million animals per year to meet the increasing U.S. demand for the drug.
Interestingly, EcoRI cuts DNA in a staggered, zig-zag manner, leaving so-called "sticky ends"; overhanging single strands that can bond with complementary sequences. Watch this video if you want to understand how restriction enzymes work more.
I have to share this fun plasmid rap!